the study
Published September 5, 2024 Author
Protein design team and wet lab team
New AI systems have the potential to design proteins that better bind to target molecules, advancing drug discovery, disease understanding, and more.
Every biological process in the body, from cell growth to immune responses, relies on interactions between molecules called proteins. Like a key in a lock, one protein binds to another and helps control important cellular processes. Protein structure prediction tools like AlphaFold have already given us tremendous insight into how proteins interact to perform their functions, but since these tools directly manipulate those interactions, cannot create new proteins.
However, scientists can create new proteins that better bind to target molecules. These binders help researchers accelerate advances in a wide range of research, including drug development, cell and tissue imaging, disease understanding and diagnosis, and even crop resistance to pests. Although recent machine learning approaches to protein design have made great strides, the process remains laborious and requires extensive experimental testing.
Today we are introducing AlphaProteo, our first AI system for designing novel high-strength protein binders to serve as building blocks for biological and health research. This technology has the potential to accelerate the understanding of biological processes and aid in the discovery of new drugs and the development of biosensors.
AlphaProteo can generate new protein binding substances for a variety of target proteins, including VEGF-A, which is associated with cancer and diabetes complications. This is the first time that an AI tool has successfully designed a protein binder for VEGF-A.
AlphaProteo also achieved higher experimental success rates and 3-300 times better binding affinities than the best existing methods for the seven target proteins tested.
Learn the intricate ways proteins bind to each other
Designing protein binders that can bind tightly to target proteins is difficult. Traditional methods are time-consuming and require extensive lab work over multiple rounds. After the binder is created, additional experiments are performed to optimize the binding affinity, so it binds tightly enough to be useful.
Trained on massive amounts of protein data from the Protein Data Bank (PDB) and over 100 million predicted structures from AlphaFold, AlphaProteo has learned the myriad ways molecules bind to each other. Given the structure of a target molecule and a set of preferred binding positions on that molecule, AlphaProteo generates candidate proteins that bind to the target at those positions.
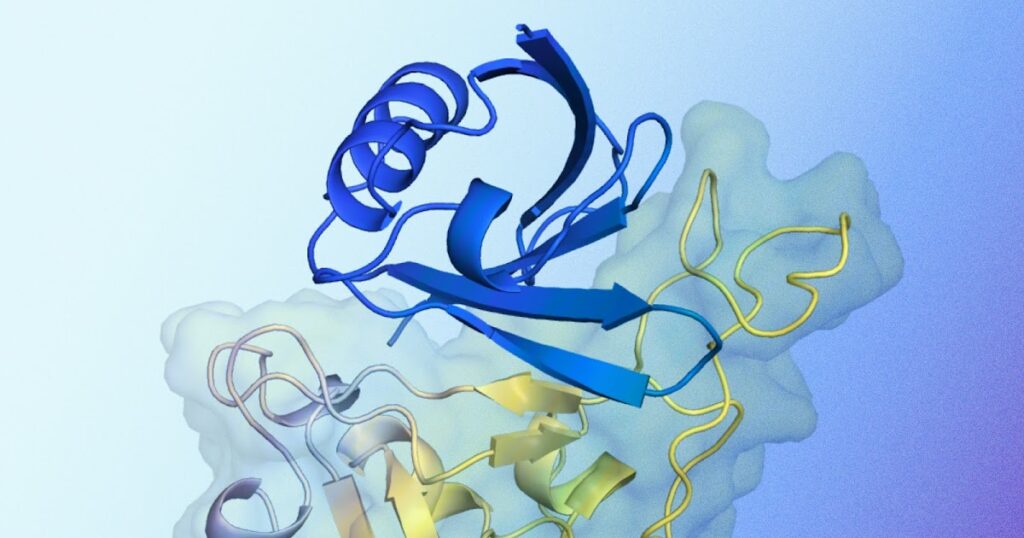
Diagram of predicted protein binder structures interacting with target proteins. Shown in blue is the predicted protein binding structure generated by AlphaProteo and designed to bind to the target protein. Shown in yellow are the target proteins, specifically the SARS-CoV-2 spike receptor binding domain.
Demonstrates success against important protein binding targets
To test AlphaProteo, we tested two viral proteins involved in infection, BHRF1 and the SARS-CoV-2 spike protein receptor binding domain, SC2RBD, and five proteins involved in cancer, inflammation, and autoimmune diseases, IL. We designed binders for a variety of target proteins, including – 7Rɑ, PD-L1, TrkA, IL-17A, VEGF-A.
Our system has a competitive binding success rate and best-in-class binding strength. For seven targets, AlphaProteo generated candidate proteins in silico that bind strongly to the protein of interest when tested experimentally.
Grid showing the predicted structures of seven target proteins for which AlphaProteo generated successful binders. Shown in blue are examples of binders tested in the wet lab, shown in yellow are protein targets, and highlighted in dark yellow are intended binding regions. is.
One specific target, the viral protein BHRF1, was successfully bound by 88% of candidate molecules when tested in Google DeepMind Wet Lab. Based on tested targets, the AlphaProteo binder binds on average 10 times stronger than existing best design methods.
For another target, TrkA, our binder is even more potent than the best binder previously designed for this target after multiple rounds of experimental optimization.
Bar graph showing the experimental in vitro success rate of AlphaProteo output for each of the seven target proteins compared to other design methods. A higher success rate means fewer designs need to be tested to find a successful binder.
The bar graph shows the best affinity of the AlphaProteo design without experimental optimization for each of the seven target proteins compared to other design methods. Lower affinity means that the binder protein binds more tightly to the target protein. Note the logarithmic scale of the vertical axis.
Validate the results
In addition to in silico validation and testing of AlphaProteo in our wet lab, we worked with the research groups of Peter Cherepanov, Katie Bentley, and David LV Bauer at the Francis Crick Institute to validate the protein binder. Through various experiments, they further investigated some of the more potent SC2RBD and VEGF-A binders. The research group confirmed that the binding interactions of these binders were indeed similar to those predicted by AlphaProteo. Furthermore, the research group confirmed that the binding agent has useful biological functions. For example, some of our SC2RBD binders have been shown to prevent SARS-CoV-2 and some of its variants from infecting cells.
AlphaProteo’s performance demonstrates that it can significantly reduce the time required for initial experiments involving protein binders of wide application. However, we know that our AI system has limitations, as we were unable to successfully design a binder for the eighth target, TNFα, a protein associated with autoimmune diseases such as rheumatoid arthritis. We selected TNFα to strongly compete with AlphaProteo because computational analysis showed that designing a binder against it would be very difficult. We will continue to improve and extend AlphaProteo’s capabilities, with the goal of ultimately addressing these challenging goals.
Achieving strong binding is usually only the first step in designing proteins with potential for practical applications, and there are many more biotechnological obstacles to overcome in the research and development process.
Towards responsible development of protein design
Protein design is a rapidly evolving technology that has implications for everything from understanding the causes of disease, to accelerating the development of diagnostic tests during viral outbreaks, to supporting more sustainable manufacturing processes, and even removing contaminants from the environment. It has a lot of potential to advance science in all aspects.
To consider potential risks in biosecurity, building on our long-standing approach to liability and safety, we collaborate with leading external experts to share this commitment and develop best practices. provides a step-by-step approach to inform community efforts. NTI’s (Nuclear Threat Initiative) New AI Bio Forum.
Going forward, we will continue to work with the scientific community to leverage AlphaProteo for impactful biological questions and understand its limitations. We are also investigating its application to drug design at Isomorphic Labs and are excited about future developments.
At the same time, we continue to improve the success rate and affinity of AlphaProteo’s algorithms, expand the range of design problems we can address, and collaborate with researchers in machine learning, structural biology, biochemistry, and other fields to improve accountability. I am developing an algorithm. Provide more comprehensive protein design for the community.
If you are a biologist whose research could benefit from target-specific protein binding and are interested in becoming a trusted tester for AlphaProteo, please contact us at alphaproteo@google.com.
Messages received will be processed in accordance with our Privacy Policy.
Acknowledgment
This research was co-developed by our protein design and wet lab teams.
We would like to thank Francis Crick Institute collaborators Peter Cherepanov, David Bauer, Katie Bentley and their group for providing valuable experimental insights and results, as well as input and evaluation of training on the initial work and algorithms. The AlphaFold team for providing their insights, and the many other teams at Google DeepMind that contributed to this program.